Use of Blunt-Tip Suture Needles to Decrease Percutaneous Injuries to Surgical Personnel is the focus of a new Safety and Health Information Bulletin (SHIB) published by OSHA and the National Institute for Occupational Safety and Health. The SHIB describes the hazards of sharp-tip suture needles and presents evidence of the effectiveness of blunt-tip needles in decreasing injuries. It also emphasizes OSHA’s requirement to use appropriate, available and effective safer medical devices.
The Occupational Safety and Health Administration’s (OSHA’s) Bloodborne Pathogens standard (29 CFR 1910.1030) protects employees against occupational exposure to bloodborne pathogens. Bloodborne pathogens are pathogenic microorganisms that may be present in human blood and cause human disease. These pathogens include, but are not limited to, hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV). Occupational exposure to bloodborne pathogens may occur when workers receive a penetrating injury to their skin (percutaneous injury). Surgical personnel are at risk of occupational exposure to bloodborne pathogens from injuries caused by sharp surgical instruments. Such personnel include surgeons, nurses, surgical technicians, anesthesiologists, and other health care personnel both inside and outside the operating suite.
Purpose
The purpose of this Safety and Health Information Bulletin is:
Background
Sharp-tip suture needles are the leading source of percutaneous injuries to surgical personnel, causing 51% to 77% of these incidents. Sharp-tip suture needles not only injure surgical staff, but also present a risk to patients from potential exposure to injured staff’s blood. Suture needle injuries can occur when surgical personnel:
Suture needle injuries frequently occur during suturing of muscle and fascia. In 2005, the American College of Surgeons (ACS) issued a statement supporting universal adoption of blunt-tip suture needles for suturing fascia and encouraging further investigation of their appropriate use in other surgical applications. The ACS stated that “all published studies to date have demonstrated that the use of blunt suture needles can substantially reduce or eliminate needle-stick injuries from surgical needles.”4 The Association of Perioperative Registered Nurses (AORN) endorsed this ACS statement in support of blunt-tip suture needle use where effective and clinically appropriate.5 The ACS and AORN are two of the seven member organizations of the Council on Surgical and Perioperative Safety, all of which have endorsed this statement. The other five member organizations are: the American Association of Nurse Anesthetists, the American Association of Surgical Physician Assistants, the American Society of Anesthesiologists, the American Society of PeriAnesthesia Nurses, and the Association of Surgical Technologists.
Engineering Controls
Engineering controls are defined in OSHA’s Bloodborne Pathogens standard as controls that isolate or remove the bloodborne pathogens hazard from the workplace [29 CFR 1910.1030(b)]. The standard states “Engineering and work practice controls shall be used to eliminate or minimize employee exposure” [29 CFR 1910.1030(d)(2)(i)]. This means that if an effective and clinically appropriate safety-engineered sharp exists, an employer must evaluate and implement it. However, if using a safer device compromises either patient safety or medical integrity its use would not be required.
Blunt–tip suture needles are identified by OSHA as an example of an engineering control to reduce percutaneous injuries (CPL 02-02-069, XIII D.2) (Citation Guidelines). As an alternative to sharp-tip suture needles, blunt-tip suture needles can be used to suture less-dense tissue such as muscle and fascia. Since as many as 59% of suture needle injuries occur during suturing of muscle and fascia, the replacement of conventional sharp-tip suture needles with blunt-tip suture needles for these surgical tasks will reduce injury rates to surgical personnel. Conventional sharp-tip suture needles may be needed to suture skin, bowel, and blood vessels, although suture-less techniques for these procedures are available for use at the discretion of the surgeon. Several case series suggest, but do not conclusively demonstrate, that blunt suture needles may also be used for suturing bowel and skin.
Evidence of Effectiveness of Blunt-Tip Suture Needles
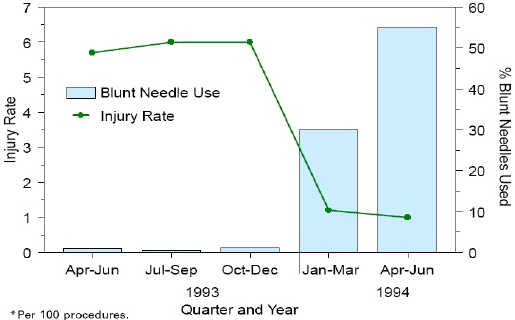
Multiple studies have reported the effectiveness of blunt-tip suture needles in decreasing percutaneous injuries. A Centers for Disease Control and Prevention (CDC) study of suture needles in gynecologic surgery found a statistically significant reduction of injury rates when blunt-tip suture needles were used (figure 1). In addition, use of these devices resulted in minimal clinically apparent adverse effects on patient care and were generally accepted by participating surgeons. Other trials and reports have shown that blunt-tip suture needles are technically satisfactory when used appropriately and that their use results in substantial reduction or elimination of injuries to surgical personnel.
Figure 1. Rate* of injury associated with use of curved suture needles
during gynecologic surgical procedures and percentage of suture needles used that
were blunt, by quarter — three hospitals, New York City, April 1993-June 1994
Conclusion
OSHA and NIOSH strongly encourage the use of blunt-tip suture needles, whenever feasible and appropriate, to decrease percutaneous injuries to surgical personnel. Clinical use and scientific studies have established the effectiveness of blunt-tip suture needles in decreasing the risk of percutaneous injuries. Employers in workplaces that use suture needles have the responsibility under the Bloodborne Pathogens standard to evaluate the use of blunt-tip suture needles as well as other appropriate safer medical devices. After this evaluation, which must include input from nonmanagerial employees responsible for direct patient care [29 CFR 1910.1030(c)(1)(v)] – e.g., in this case surgical personnel – employers must use safer devices to replace corresponding conventional sharp-tip suture needles in their workplaces when clinically appropriate. The introduction of any device must include training of staff in its proper use and follow-up to ensure a successful transition from conventional to safer devices. Where an employer has determined that the use of available safer devices is not feasible, the clinical justification for this determination must be documented in the facility’s Exposure Control Plan and the employer must implement alternative means of protecting surgical personnel from percutaneous injuries.
Additional Tools and Information
Additional information and resources on bloodborne pathogens and percutaneous injury prevention, including the complete text of OSHA’s Bloodborne Pathogens standard and the Needlestick Safety and Prevention Act, are available on OSHA’s Safety and Health Topics Page at and on NIOSH’s Safety and Health Topic Page: Bloodborne Infectious Diseases HIV/AIDS, Hepatitis B Virus, and Hepatitis C Virus. OSHA’s web site contains extensive resources on workplace safety and health as well as contact information for asking OSHA a question or filing a workplace safety and health complaint. NIOSH’s web site also contains extensive resources on workplace health and safety and contact information, including access to CDC’s Workbook for Designing, Implementing, and Evaluating a Sharps Injury Prevention Program. The Workbook contains detailed information on how to select and evaluate sharps devices with engineered sharps injury prevention features as well as a sample device evaluation form.